Microfluidic-Based Beta-Cell Functional Analysis Facility
The Microfluidic-based beta-cell functional analysis facility is located in the Department of Surgery, at the University of Virginia (UVA) and is supported by the Juvenile Diabetes Research Foundation International (JDRF).
This functional analysis facility
- The overall purpose is to assist the field of diabetes research in moving forward with therapeutic strategies for beta-cell replacement and beta-cell regeneration to cure Type I diabetes.
- The facility provides: Analysis and characterization services for emerging islet cell derivatives including hESCs/hiPSCs, and microencapsulated islets/hESCs/hiPSCs.
- The facility provides: Screening of potential pharmaceutical agents for diabetes treatment.
- The analysis of hESCs/hiPSCs islet cell derivatives become available to the JDRF researchers upon an approval by JDRF.
- All services provided by the Facility will be available to diabetes researchers who may not have such technology or access, based on pay-for-service.
- Additionally, we are also able to service those who are working on islet or beta-cell related research.
Islet cell derivatives are analyzed to answer the following basic questions
- Compared to human islets, do the biologics have similar physiological response profiles in the presence of glucose or other secretagogues?
- Physiologically, what are the functional similarities and/or dissimilarities of the biologics to human islets?
- Does the biologics have normal or altered insulin stimulator-secretion coupling factors and what, if any, is the impact of pathophysiological changes on insulin secretion?
- How does microencapsulation or hypoxia impact the function and survival of microencapsulated islet cells?
Use the Service Request Form (PDF) to initiate services. Or, contact the Analysis Facility directly.
More information about the analysis facility
In 2009, we designed a three-layer microfluidic perifusion device specifically for pancreatic islets of Langerhans (Fig.1A). This initial design has evolved into a family of microfluidic devices that can be applied to the study of islet physiology and pathophysiology.
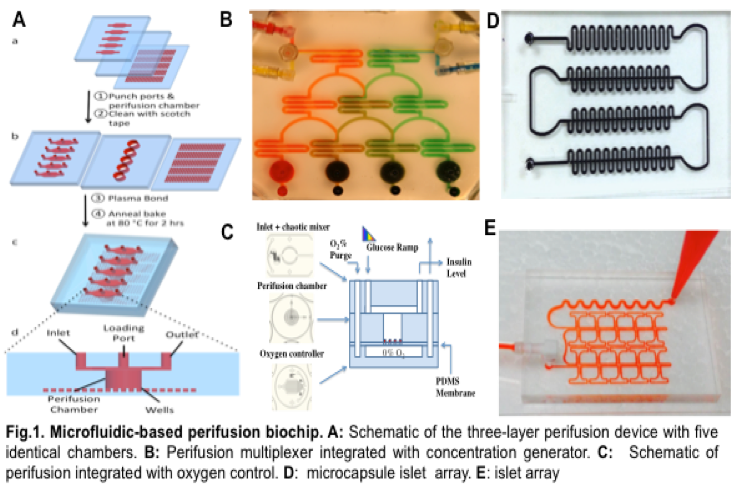
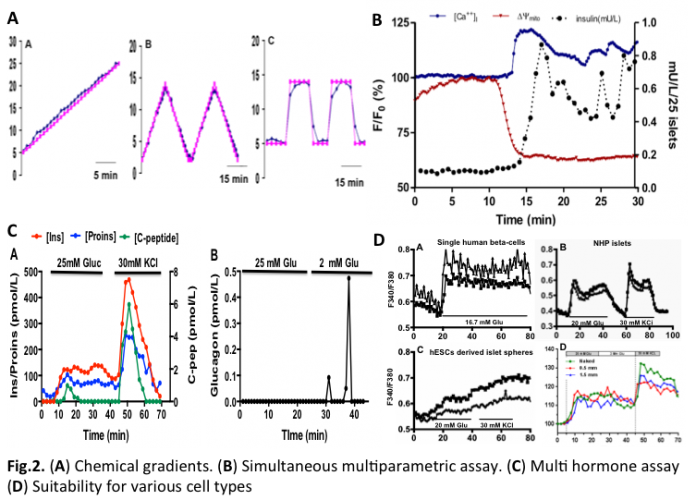
In the current system, islet fixation is not required. Specific chemical gradients can be established and well maintained in the perifusion chamber (Fig.2A). The microfluidic perifusion system measures not only insulin secretion kinetics but also insulin stimulator-secretion coupling factors by integrating with multiparametric optical imaging technology, including measurement of calcium influx, mitochondrial potentials, NAD(P)H, ROS, glucose uptake (individually or combined two or three simultaneous fluorescence imaging) (Fig2B). In addition to insulin measurements, additional islet hormones can be measured with tested spatiotemporal resolution including; proinsulin, C-peptide, and glucagon (Fig.2C). Furthermore, various islet types, single beta-cells, and encapsulated islet cells can be analyzed.
Service Request Form
We propose the following array of multiple assays with their associated rationale, as well as a decision tree/order of the proposed assays.
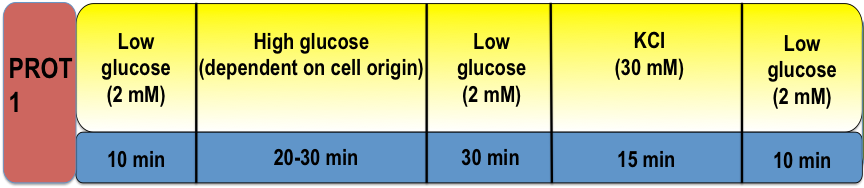
Protocol 1 – Testing Physiological Function and Viability by Pulsed Glucose and KCI Stimulation
We first test the functionality of the cell sample by simple pulse stimulation with a high glucose concentration, followed by 30 mM KCI. From this protocol, we can characterize the physiological similarities or differences of cell-biologics compared to human islets. Furthermore, it acts as an initial test criteria to determine if further testing is needed. If the cell-biologic passes the initial test, the following systematic protocols may follow to further interrogate their insulin secretory pathway.
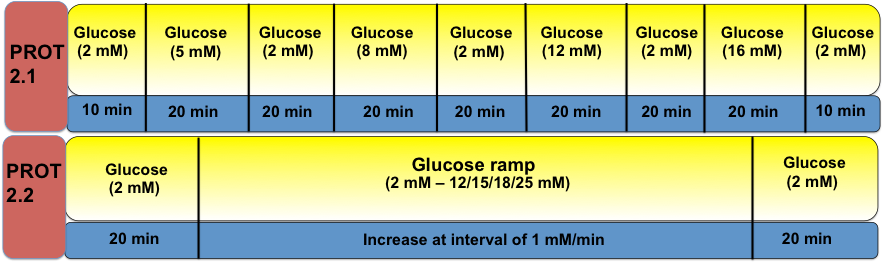
Protocol 2 – Determination of Optimal Glucose Sensitivity using Incremental Step-Up Glucose Pulse Stimulation and/or Glucose Ramp Stimulation
It is known that some cell derivatives respond to glucose differently when compared to human islets, for example glucose sensitivity shift. This shift in glucose sensitivity is evidence that the cells are responsive to glucose either at lower or higher glucose concentrations when compared to human islets. It is expected that the iBCs derived from pluripotent stem cells might initially form cells that display a variation in glucose sensitivity, due in part to its position as a developmental intermediate. It is important not only to address how this varying threshold may change during maturation, but also to prevent potential complications such as hypoglycemia, which will have profound effects on clinical outcomes.
Step-up glucose pulse stimulation provides useful information on whether a shift in glucose sensitivity occurs. However, a detailed change in threshold/sensitivity can be further verified by a glucose ramp over a narrowed range of glucose concentrations determined by step-up glucose pulse experiments.
Protocol 3. Dissection of Insulin Stimulator-Secretion Coupling Factors
Glucose-induced beta-cell insulin secretion is a cascade that is mainly mediated through the KATP-dependent pathway. This pathway involves several key stimulator-secretion coupling factors. Any defect or change in these coupling factors will significantly impact insulin secretion and, subsequently, in vivo function. Many iBCs are acknowledged to respond to glucose differently when compared to normal human islets. This difference may result from a complete absence of or immaturity of stimulator-secretion coupling factors through the differentiation protocols employed. Additionally, glucose can increase cytoplasmic calcium concentrations by inducing calcium release from the endoplasmic reticulum (ER) calcium stores without biphasic and pulsatile signatures. Often, the ER-induced calcium stimulates small quantities of insulin release in a non-controlled manner. Additionally, this can provide misleading information on iBCs glucose-responsiveness and functional maturity.
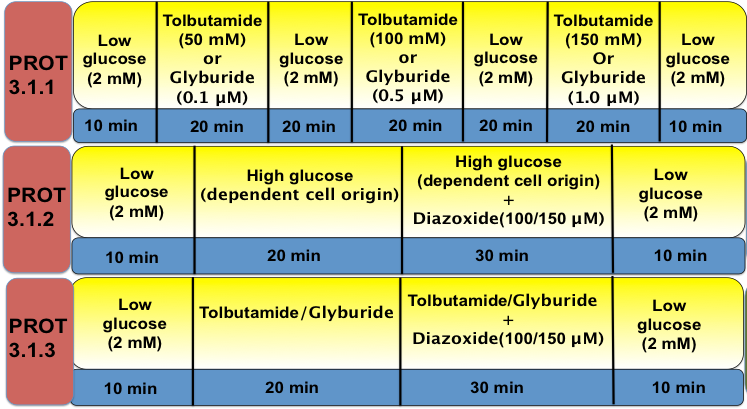
Protocol 3.1 Verification of KATP Channel Activity
Since both plasma KATP channel existence and activity play an important role in glucose-induced insulin secretion, the KATP channel activity is assessed with dose responses of KATP channel closers (Tolbutamide and/or glyburide). Additionally, KATP channel activity can be further verified by KATP channel openers (Diazoxide) in response to either glucose or KATP channel closers.
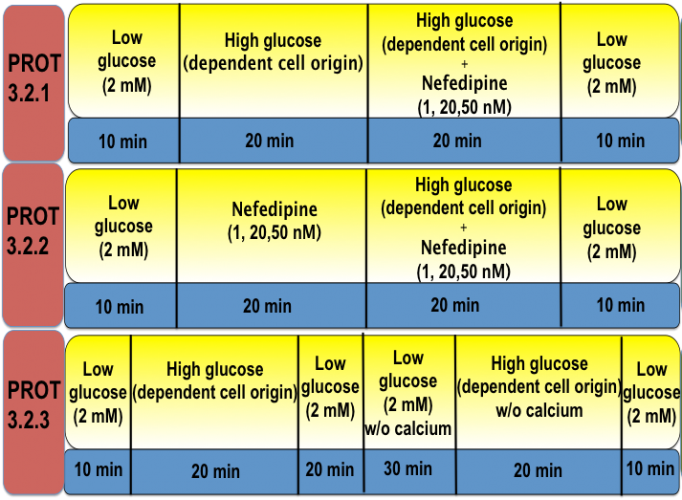
Protocol 3.2 Verification of VDCCs Activity
Glucose-induced calcium influx mainly occurs through the voltage-dependent calcium channels (VDCCs). We have observed that glucose-induced calcium may be independent of VDCCs in some iBCs. This may explain why iBCs often have abnormal insulin secretion patterns and lower insulin release in response to the glucose challenge. Here, we apply Nifedipine (L-type Ca2+ channel closer) pre or post glucose administration to determine the spatiotemporal relationship between [Ca2+]I and insulin secretion in cells to be tested. Additionally, we can determine the origin of cytoplasm calcium by using a calcium-free Krebs-Ringer buffer and assessing [Ca2+]i in response to glucose.
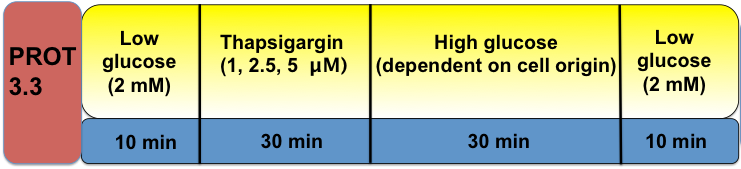
Protocol 3.3 Determination of Intracellular Calcium Source
In addition to activation through the VDCCs, cytoplasm calcium can also be induced by glucose through calcium release from the ER. This in turn then triggers insulin secretion; however, such calcium signaling often does not have normal biphasic and oscillatory patterns, similar to VDCC-regulated calcium signaling. In this protocol, thapsigargin (an ER calcium ATPase pump blocker) is applied to deplete ER calcium before glucose stimulation in order to determine the role, if any, that ER calcium plays in insulin secretion and intracellular signaling of test cell derivatives.
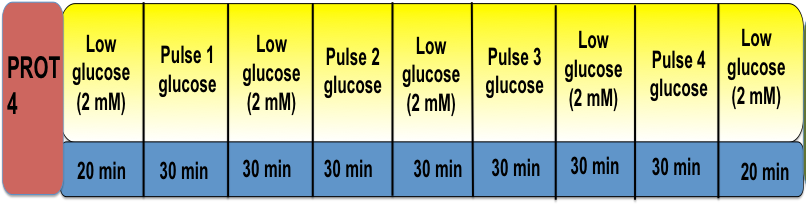
Protocol 4. Determination of Maximal Insulin Secretion Capability and Exhaustion
Beta-cell insulin granules populate two different pools: the readily releasable pool (RRP) and the reserved pool (RP). The RRP pools are associated with phase I secretion. In contrast, phase II secretion requires the trafficking of the RP to the plasma membrane. Normal beta-cells contain excess amounts of insulin granules and only a small percentage (1-5%) is utilized for insulin secretion under each stimulation. We apply repetitive glucose stimulation to mimic in situ glucose homeostasis to determine maximal insulin secretion capabilities and identify insulin secretion defects in phase I/II or beta-cell exhaustion. This pulse stimulation protocol may provide useful information on insulin granule distribution and release capability (RRP pools vs. reserved pools) by analyzing phase I vs. phase II or phase I or II vs. total insulin release. If repeated stimulation elicits a significant reduction of acute insulin release with each pulse then the beta-cells are considered exhausted. This in turn indicates that the cell derivative may not provide long-term in vivo function.
Protocol 5. Determination of the Impact of Microencapsulation
Microencapsulation is a promising approach to prevent islet graft immunorejection. It is important to understand the impact of the encapsulation process, capsule material, and capsule size on the nutrient/insulin diffusion, glucose metabolism, and function of encapsulated cells. Additionally, hypoxia is considered another important factor responsible for the functional loss of encapsulated islets. Encapsulation aggravates islet hypoxia by preventing revascularization and increases the oxygen diffusion distance. Hypoxia may also facilitate the attraction of macrophages and, subsequently, causes cell overgrowth on the microcapsule.
The function of microencapsulated islets, impact of capsule materials, and capsule sizes are assessed using previously described protocols (1-4). For the hypoxia study, microencapsulated islets or islet-like cells are assessed using the perifusion device integrated with an oxygen controller shown in Figure 1C.
If required by interested researchers, we can further provide new experimental protocols focused on the following areas: (please note that all protocols are well established and fully verified).
- Simultaneous measurement of [Ca2+]i/NAD(P)H and insulin.
- Simultaneous measurement of ROS using DCFDA/MitoSox, [Ca2+]i, and insulin.
- Investigation of insulin secretion independent of KATP channels and amplification of KATP-dependent pathways through the application of GLP-1, carbachol, glucose + arginine, or glucose + amino acid mix.
- Investigation of long-term dynamic and dose-response of pharmaceutical agents on islet survival and function using the biochip shown in Figure 1C.
- High-throughput, high-content imaging, and screening of iBCs and pharmaceutical agents using the array biochip (Fig. 1D and E) that can hold up to 200 islets or encapsulated islets in an array.
The UIC Human Islet Transplant Program has a certified cGMP isolation facility and has conducted more than 600 human islet isolations. The program is one of only six centers funded through the IIDP for the distribution of human islets. Additionally, we have archived data from over 150 human islet preparations using this microfluidic assay, as well as in vivo animal transplant data, histological imaging/analysis, and other in vitro data from each preparation that is useful as an historical control. Since we isolate human islets on a weekly basis, freshly isolated human islets can be used as a suitable control whenever required by cell providers.
- All data gathered from performed analysis will be held and treated as highly confidential. The data will only be communicated between “cell providers” and “test performers” as required by JDRF. “Test performers” have absolutely no rights to publish or publicly communicate the data without prior written permission from “cell providers”.
- This facility is established based on a pay-per service agreement with JDRF for JDRF sponsored research projects. We are willing to work together with interested researchers to adapt the microfluidic technology and develop any new protocol for optimal analysis of their cells and purposes.
- Cell derivatives tested should preferably be shipped under the cold shipping protocol (~8ºC) adopted from NIH-sponsored IIDP at a maximal density of 250 islets or equivalent/mL. Given the different cell-biologic cell source origins, it is the responsibility of cell providers to determine optimal culture media, as well as the antibiotics/growth factors to be used and communicate with test performers. The Cell-biologic providers should provide extra culture media to “test performers”. Cell providers should provide any necessary information on cell origin, type, cell amount, and protocols to be performed. It is also the responsibility of cell providers to provide all necessary biohazard information. All cell derivatives are preferably shipped in suspension. If shipped in adherent status, cell providers must provide the necessary information and protocols for cell handling. The detailed information of shipping protocol and cell quantity will be provided upon request by researchers.
- The cost of analysis for the interested researchers outside JDRF sponsored projects is depended on the protocol selected and extent of the analysis requested. For detailed information, please contact the facility director prior to analysis.
Service Request Form
| Cells/Materials | Parameters | Characterization and Quantification | Control |
|---|---|---|---|
|
Calcium signaling [Ca2+]i |
|
Human islets |
| Mitochondrial potential (ΔѰM) |
|
Human islets | |
| Hormone secretion kinetics |
|
Human islets | |
|
[Ca2+]i ΔѰM Hormone kinetics | Same as above |
|
Analysis Facility Contacts
Department of Surgery
The University of Virginia
Carter Harrison Bldg (MR6) Rm B711
345 Crispell Dr.
Charlottesville, VA 22908
